Co-Moderator at the AI Summit 2023 hosted by the AFDO/RAPS Healthcare Products Collaborative, Cincinnati, 16 NOV 2023
Managing the AI Software Development Life Cycle
Discussed how introducing AI/ML into the SDLC changes things.
Guest Panelist at the CoinGeek Conference New York, Sheraton New York Times Square, 06 OCT 2021
Health Care, Life Sciences & Blockchain
(Click link for video - contribution starts at 1:33:16 and runs to ~2:04:05) The presenter shared personal interest in Bitcoin to realize disruption to existing operating models in life sciences as a critical success factor for data integrity solutions.
Guest Panelist at the Graduate and Postdoctoral Career Day at Penn State College of Medicine, Hershey PA, 19 OCT 2019
Business Track, Consulting Panel
The 10th Biennial Career Day was a day-long educational & networking event for graduate students and postdoctoral fellows to learn about a wide range of career options and experiences available in the biomedical sciences and public health. The day was divided into four career tracks with approximately 14 to 16 panels.
Guest Speaker for closing Plenary Session at [inform] 2019, Waters Informatics Users’ Conference, Sheraton Austin Hotel at the Capitol, 23 MAY 2019
Regulatory Guidance & Recommendations for Data Integrity
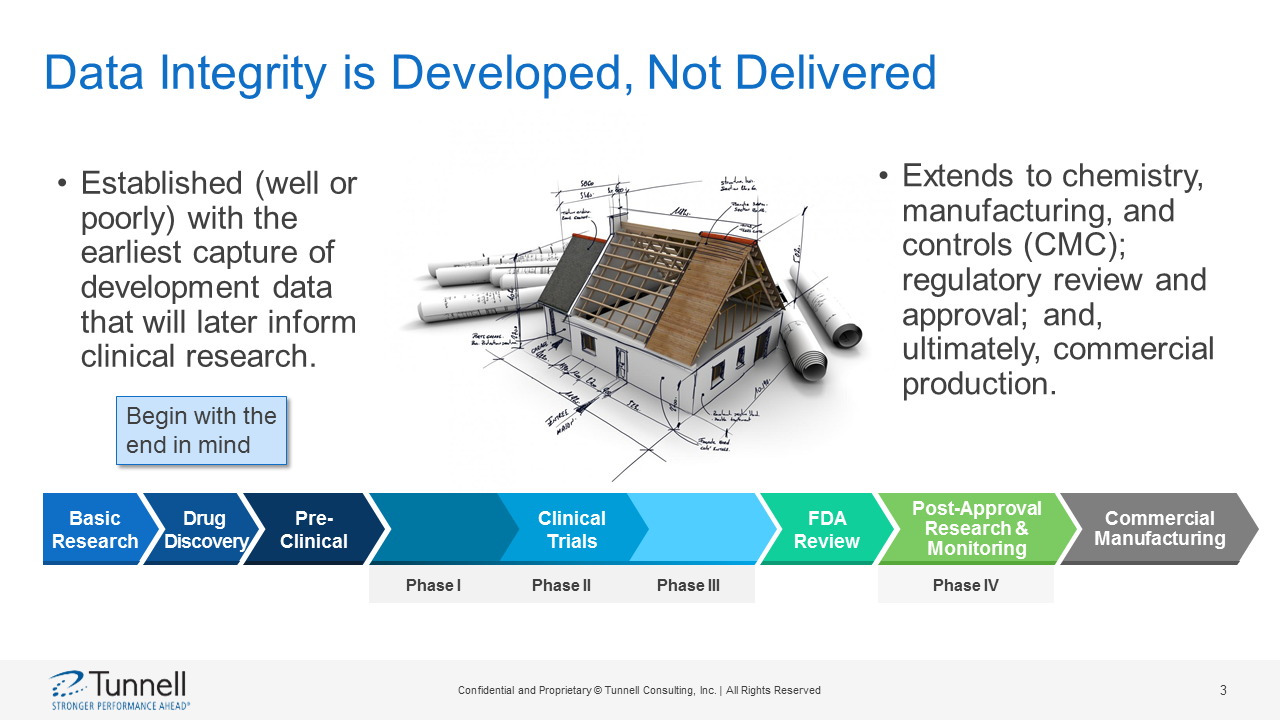
Hear how regulations and regulatory guidance increasingly focus on data integrity; and, how human behavior remains at the center of health authority attention. While laboratory processes and supporting technology systems have matured over the years, human behavior continues to present challenges. Don’t let your actions negatively affect data integrity. Good data integrity is developed, not delivered; and, is established (well or poorly) with the earliest capture of development or laboratory data that will later inform clinical research and down-stream commercial manufacturing activities.
Speaker at Data Integrity & Pharmaceutical Quality Compliance Summit, Metro Meeting Center, Boston, MA, 04 MAR 2019
Regulatory Guidance for Data Integrity
The presenter described the various regulatory and guidance documents that exist related to data integrity in the life sciences industry and provided examples of the current regulatory compliance landscape from contemporary inspection results. The presenter also provided specific case study examples of compliance remediation related to data integrity.
Panelist at Life Science Vendor Exhibit (LSVE), New York Genome Center, 15 OCT 2018
Product Development to Project Management: Lessons Learned
Why are product road maps important and what goes into creating product development plans? How these will help you get investors and assure you are on the right track.
Moderated panel for “Fundamentals for Outsourcing Drug Development and Manufacturing” at Outsourced Pharma Conference, Philadelphia, PA. 19 JUL 2018
Make Outsourcing More About Patients
Does the outsourcing of drug development and manufacturing only benefit sponsors and service providers? What about the actual patients? Sponsors and their CDMOs should challenge each other to better serve and connect the patient to the outsourcing model. In fact, this panel of experts demonstrates how doing so can helps ensure the success of everyone involved.
Moderated panel for “Fundamentals for Outsourcing Drug Development and Manufacturing” at Outsourced Pharma Conference, Philadelphia, PA. 18 JUL 2018
Negotiating Strategies For Quality And Supply Agreements
This session unfolds in three acts. First, learn strategies for negotiating your quality agreement. Next, find out how to better negotiate your supply agreement. Finally, and most important, learn how to ensure both agreements are consistent and actually work together to achieve your – and your provider’s – goals.
ISPE GAMP Data Integrity SIG Knowledge Sharing Session, online/WebEx. 14 JUN 2018
Data Integrity Practices Along the Product Life Cycle
The presenter will describe how data integrity is established (well or poorly) with the earliest capture of development data that will later inform clinical research. It then extends to chemistry, manufacturing, and controls (CMC); regulatory review and approval; and, ultimately, commercial production. Specific examples of data integrity opportunities will be presented along the product development life cycle with real-world examples of pitfalls to avoid.
ISPE 2017 Annual Meeting & Expo, Information Systems Track, San Diego, CA. 31 OCT 2017
Information Governance for Data Integrity
Data Integrity issues are at the forefront of inspection observations and a common citation within FDA Warning Letters. Innovative thinking and non-traditional approaches to Information Governance present not only improved Data Integrity but true competitive advantage when applied as a transformation initiative with cultural change as an intended result. Learn how to apply creative design to governance, simplified operational excellence and risk management approaches to information and data challenges, and clever change management techniques to break down silos and realize truly transformation change.